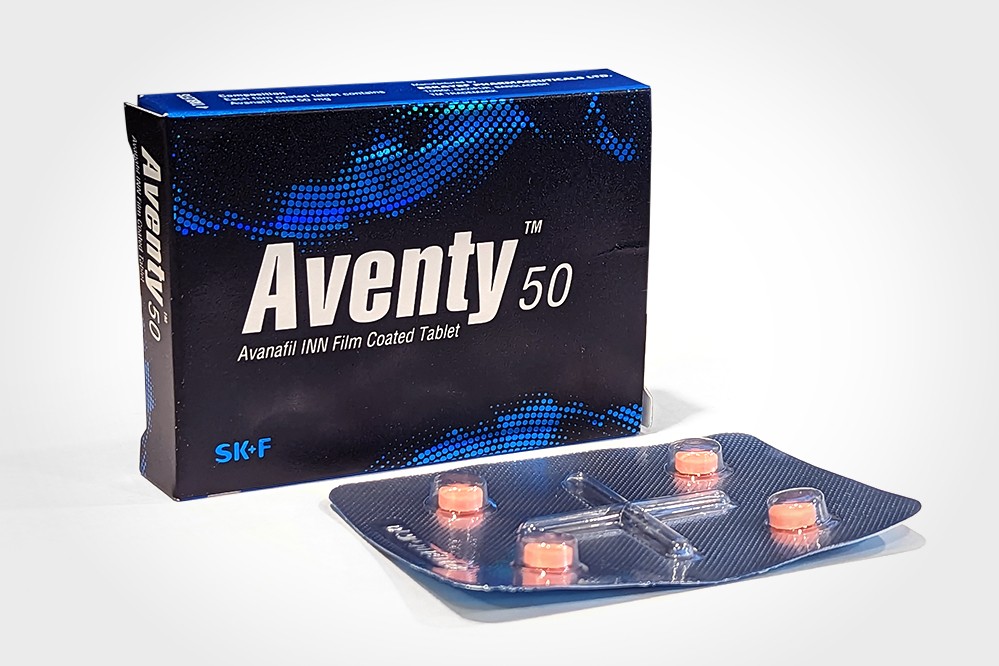
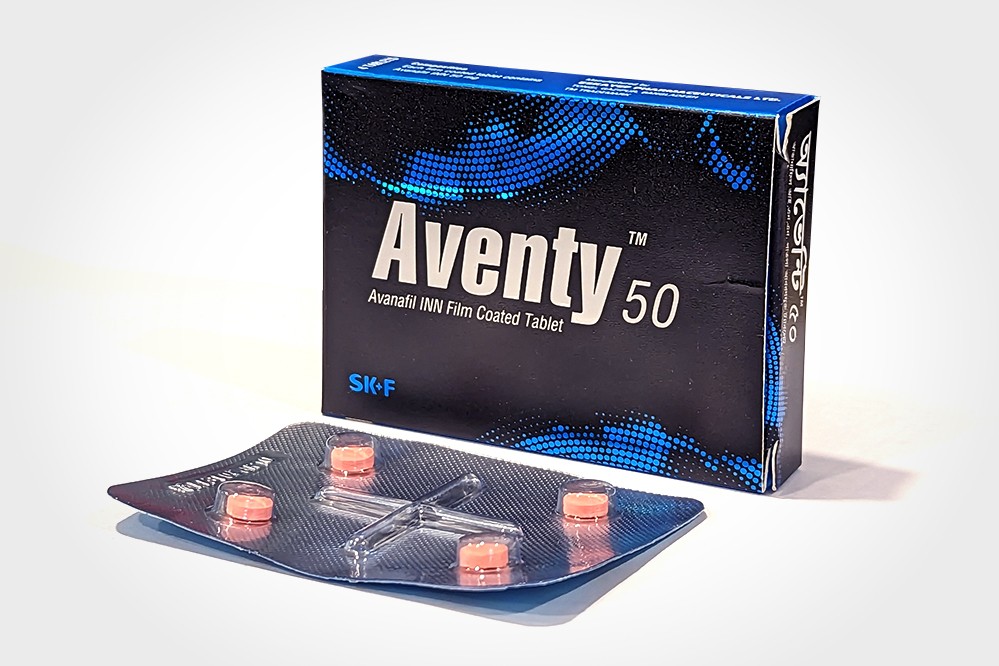






Aventy
DESCRIPTION
AventyTM is a selective phosphodiesterase 5
(PDE5) enzyme inhibitor used for the treatment of erectile dysfunction caused
by diabetes, age induced oxidative stress or other complications. Avanafil
inhibits the cGMP specific phosphodiesterase 5 (PDE5) which is responsible for
degradation of cGMP in the corpus cavernosum located around the penis. Penis
erection during sexual stimulation is caused by increased penis blood flow
resulting from the relaxation of penis arteries and corpus cavernosal smooth
muscle. This response is mediated by the release of nitric oxide (NO) from
nerve terminals and endothelial cells, which stimulates the synthesis of cGMP
in smooth muscle cells. Cyclic GMP causes smooth muscle relaxation and
increased blood flow into the corpus cavernosum. The inhibition of
phosphodiesterase 5 (PDE5) by avanafil enhances erectile function by increasing
the amount of cGMP.
INDICATION
AventyTM is a phosphodiesterase 5 (PDE5)
inhibitor indicated for the treatment of erectile dysfunction.
DOSAGE AND ADMINISTRATION
The recommended starting dose is 100 mg taken approximately
15 minutes before sexual activity, on an as needed basis. AventyTM
may be taken with or without food. AventyTM must not be taken more
than once a day. The dose may be increased to 200 mg or decreased to 50 mg
based on efficacy and/or tolerability. Use the lowest dose that provides
benefit. Concomitant use of nitrates in any form is contraindicated. Do not use
AventyTM with strong CYP3A4 inhibitors. If taking a moderate CYP3A4
inhibitor, the dose should be no more than 50 mg in a 24-hour period. In
patients on stable alpha-blocker therapy, the recommended starting dose of
Avanafil is 50 mg
CONTRAINDICATIONS
Administration of AventyTM to patients using any
form of organic nitrate is contraindicated. AventyTM is
contraindicated in patients with a known hypersensitivity to Avanafil.
Administration of AventyTM with guanylate cyclase (GC) stimulators
such as riociguat and vericiguat is contraindicated.
SIDE EFFECTS
Most common adverse reactions (greater than or equal to 2%)
include headache, flushing, nasal congestion, nasopharyngitis, and back pain.
PRECAUTION AND WARNING
Patients should not use AventyTM if sexual
activity is inadvisable due to cardiovascular status or any other reason. Use
of AventyTM with alpha-blockers, other antihypertensives, or
substantial amounts of alcohol (greater than 3 units) may lead to hypotension.
Patients should seek emergency treatment if an erection
lasts greater than 4 hours.
Patients should stop taking AventyTM and seek
medical care if a sudden loss of vision occurs in one or both eyes, which could
be a sign of Non-Arteritic Ischemic Optic Neuropathy (NAION). Discuss with
patients the increased risk of NAION in patients with a prior history of NAION.
Patients should stop taking AventyTM and seek
prompt medical attention in the event of sudden decrease or loss of hearing.
USE IN PREGNANCY AND LACTATION
Pregnancy Category C. AventyTM is not indicated
for use in women.
USE IN SPECIAL POPULATION
Pediatric Use: AventyTM is not indicated for use
in pediatric patients.
Geriatric Use: Of the total number of subjects in clinical
studies of AventyTM, approximately 23% were 65 and over. No overall
differences in efficacy and safety were observed between subjects over 65 years
of age compared to younger subjects; therefore, no dose adjustment is warranted
based on age alone. However, a greater sensitivity to medication in some older
individuals should be considered.
Renal & hepatic impairment patients: Do not use in
patients with severe renal & hepatic impairment.
STORAGE CONDITION
Store below 25 °C temperature. Keep away from light and wet
place. Keep out of reach of children.
PACKAGING
AventyTM 50 Tablet: Box containing 1 strip of 4
tablets. Each tablet contains Avanafil INN
50 mg.
AventyTM 100 Tablet: Box containing 1 strip of 4
tablets. Each tablet contains Avanafil INN
100 mg.