

DESCRIPTION
Cetrolix™ is a preparation of Cetrorelix Acetate. Cetrorelix Acetate is a synthetic decapeptide with gonadotropin-releasing hormone (GnRH)
antagonistic activity. Cetrorelix Acetate blocks the effects of GnRH. GnRH controls the secretion of luteinizing hormone (LH), which induces ovulation
during the menstrual cycle. During hormone treatment for ovarian stimulation, premature ovulation may lead to eggs that are not suitable for
fertilization. Cetrorelix Acetate blocks such undesirable premature ovulation. GnRH induces the production and release of luteinizing hormone (LH)
and follicle stimulating hormone (FSH) from the gonadotropic cells of the anterior pituitary. Due to a positive estradiol (E 2) feedback at midcycle,
GnRH liberation is enhanced resulting in an LH-surge. This LH-surge induces the ovulation of the dominant follicle, resumption of oocyte meiosis and
subsequently luteinization as indicated by rising progesterone levels.
Cetrorelix Acetate competes with natural GnRH for binding to membrane receptors on pituitary cells and thus controls the release of LH and FSH in a
dose-dependent manner. The onset of LH suppression is approximately two hours with the 0.25 mg dose. This suppression is maintained by
continuous treatment and there is a more pronounced effect on LH than on FSH. An initial release of endogenous gonadotropins has not been
detected with Cetrorelix injection, which is consistent with an antagonist effect.
The effects of Cetrorelix Acetate on LH and FSH are reversible after discontinuation of treatment. In women, Cetrorelix Acetate delays the LH-surge,
and consequently ovulation, in a dose-dependent fashion. FSH levels are not affected at the doses used during controlled ovarian stimulation. A dose
of Cetrorelix Acetate 0.25 mg every 24 hours has been shown to maintain the effect.
INDICATIONS
Cetrorelix Acetate is indicated for the treatment of infertility in females. It restricts eggs to release directly and prevents premature ovulation.
In Female infertility, Cetrorelix Acetate prevents release of premature eggs during a process called ovulation in females. This helps in normal
development of an egg in a woman's ovary (female reproductive organ), and stimulates the release of a healthy, matured egg. This helps to treat
infertility in women and increases the chance of a successful pregnancy.
DOSAGE AND ADMINISTRATION
Cetrolix™ administered subcutaneously once daily (0.25 mg dose) at 24 hour intervals, either in the morning or in the evening as part of the multiple
dose protocol during the early- to mid-follicular phase (Day 5/6 to Day11).
It is for subcutaneous injection into the lower abdominal wall. The first administration of Cetrolix™ 0.25 mg should be performed under the
supervision of a physician.
The reconstituted product is to be administered subcutaneously. Use immediately after reconstitution.
CONTRAINDICATIONS
• Hypersensitivity to cetrorelix acetate, extrinsic peptide hormones or mannitol.
• Pregnancy and lactation.
• Postmenopausal women.
• Patients with moderate and severe renal and hepatic impairment
ADVERSE REACTIONS
The most commonly reported adverse effects are local injection site reactions such as erythema, swelling and pruritus that are usually transient in
nature and mild in intensity.
Mild to moderate ovarian hyperstimulation syndrome (OHSS) (WHO grade I or II) have been commonly reported and should be considered as an
intrinsic risk of the stimulation procedure.
PRECAUTIONS AND WARNINGS
• Allergic conditions: Cases of allergic/pseudoallergic reactions, including life-threatening anaphylaxis with the first dose have been reported. Special
care should be taken in women with signs and symptoms of active allergic conditions or known history of allergic predisposition. Treatment with
Cetrorelix Acetate is not advised in women with severe allergic conditions.
• Ovarian Hyperstimulation Syndrome (OHSS): During or following ovarian stimulation, an ovarian hyperstimulation syndrome can occur. This event
must be considered as an intrinsic risk of the stimulation procedure with gonadotropins. An OHSS should be treated symptomatically, e.g. with rest,
intravenous electrolytes/colloids and heparin therapy. Luteal phase support should be given according to the reproductive medical centre´s practice.
• Repeated ovarian stimulation procedure: There is limited experience up to now with the administration of Cetrorelix Acetate during a repeated
ovarian stimulation procedure. Therefore, Cetrorelix Acetate should be used in repeated cycles only after a careful benefit/risk evaluation.
• Congenital anomalies: The prevalence of congenital anomalies after the use of assisted reproductive technologies (ART) with or without GnRH
antagonists may be slightly higher than after spontaneous conceptions, although it is unclear whether this is related to factors inherent to the
couple's infertility or the ART procedures. Limited data from clinical follow-up studies in 316 newborns of women administered Cetrorelix Acetate
for infertility treatments suggest that Cetrorelix Acetate does not increase the risk of congenital anomalies in the offsprings.
• Hepatic impairment: Cetrorelix Acetate has not been studied in patients with hepatic impairment and caution is therefore warranted.
• Renal impairment: Cetrorelix Acetate has not been studied in patients with renal impairment and caution is therefore warranted. Cetrorelix Acetate
is contraindicated in patients with severe renal impairment.
USE IN PREGNANCY AND LACTATION
Cetrorelix Acetate is not intended to be used during pregnancy and lactation.
DRUG INTERACTION
No formal drug-drug interaction studies have been performed with Cetrorelix Acetate. In vitro investigations have shown that interactions are unlikely
with medicinal products that are metabolized by cytochrome P450 or glucuronised or conjugated in some other way. However, the possibility of
interactions with gonadotropins or medicinal products that may induce histamine release in susceptible individuals, cannot be totally excluded.
SPECIAL PRECAUTION:
• This medicinal product must be allowed to reach room temperature prior to injection. It should be removed from the refrigerator approximately 30
minutes before use.
• Do not use if the reconstituted solution contains particles or if the solution is not clear.
• The solution should be used immediately after reconstitution.
PHARMACEUTICAL PRECAUTION
To be used immediately after reconstitution. Store in a refrigerator (2°C to 8°C temperature). Do not freeze. Keep away from light & wet place. Keep
out of reach of children.
PREPARATION
1. Wash your hands well with soap and water.
2. On a clean surface, lay out everything you need: one vial of Cetrolix™, one ampoule of WFI, one disposable syringe with light brown (26G)
injection needle, one green (21G) injection needle and two alcohol pads.
3. Flip off the plastic cover of the Cetrolix™ vial. Clean the aluminum ring and rubber stopper with an alcohol wipe. Discard the alcohol pad.
4. Take the disposable syringe, remove the wrapping and remove the attached light brown (26G) syringe needle and put the needle on a clean
surface.
5. Take the green (21G) injection needle, remove the wrapping and put this green needle on the syringe.
6. Take 1 mL of WFI from the ampoule using the syringe.
7. Push the needle through the center of the rubber stopper of the vial of Cetrolix™. Inject the water from the syringe into the vial by slowly pushing
down the plunger of the syringe. Dissolve Cetrolix™ powder only with the water contained in the syringe.
8. Without removing the needle from the vial, gently shake or rotate the vial until the solution is clear and without particles. Avoid forming bubbles.
DO NOT USE IF SOLUTION APPEARS CLOUDY, LUMPY or DISCOLOURED.
9. Draw all of the liquid in the vial into the syringe. If the liquid is left in the vial, invert the vial, pull back the needle until the opening of the needle is
just inside the stopper. If you look from the side through the gap in the stopper, you can control the movement of the needle and the liquid. It is
important to withdraw the entire contents of the vial. Be careful not to pull the plunger out of the syringe.
10. Detach the green needle from the syringe and put the light brown (26G) injection needle on the syringe and remove the cover of the needle.
11. Invert the syringe and push the plunger until all the air bubbles have been pushed out. Do not touch the needle or allow the needle to touch any
surface.
ADMINISTRATION
1. Choose an injection site on your lower abdominal area. It should be preferably around, but at least one inch away, from your belly button. Choose a
different injection site each day to minimize local irritation. Take the second alcohol wipe, clean the skin at the injection site and allow the alcohol
to dry. Keep the alcohol wipe nearby.
2. Pick up the syringe. Invert the syringe and hold it as if “throwing a dart”. With your other hand, gently squeeze the skin together to make a little
elevation at the injection site. Using a “dart like motion”, slowly insert the needle at an angle of about 45° to 90° (you need very little force but
quick action).
3. Once the needle is inserted into the tissue all the way, inject the solution. Do this by pushing gently on the plunger with your thumb of the hand
holding the syringe. Take as much time as you need to inject all the solution.
4. Immediately withdraw the needle. Clean the injection site with the clean side of the alcohol pad using a circular motion. If there is minor oozing,
you may need to apply a small amount of pressure for a minute.
5. Use the syringe and needles only once. Dispose of the syringe and needles immediately after use. Discard into a disposal container or
puncture-proof container with a lid that fits firmly.
OVERDOSE:
If you think you have taken too much Cetrolix™, contact your healthcare professional, hospital emergency department or regional poison control
centre immediately, even if there are no symptoms.
MISSED DOSE:
If you miss a dose of Cetrolix™, do not take a double dose. Discuss with your doctor when you should receive your next dose. Check with your
doctor if you have any questions about this.
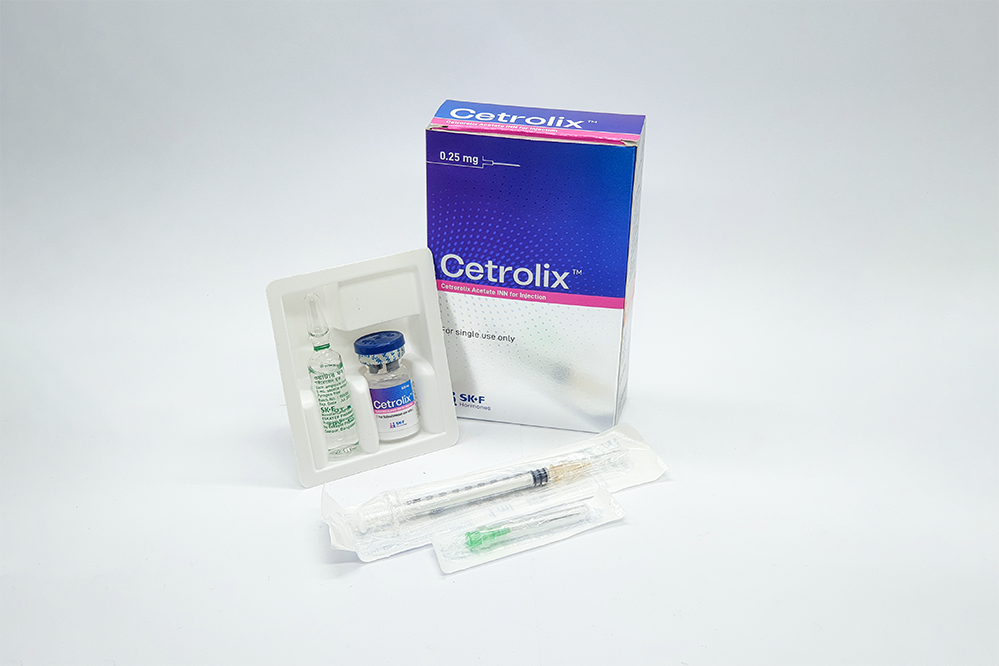
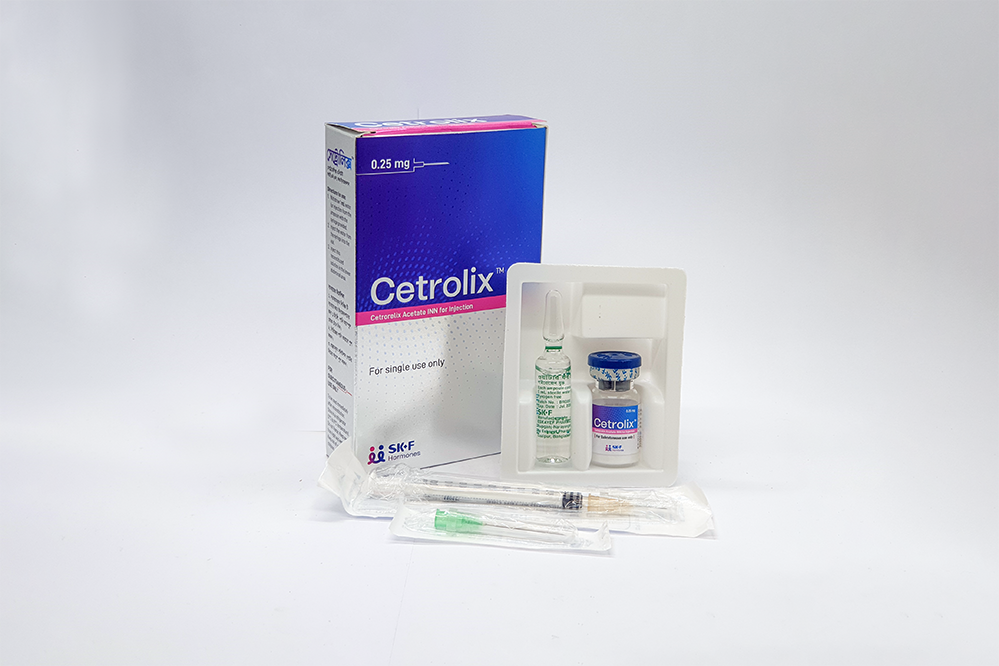
PACKAGING
Each box of Cetrolix™ contains:
• One vial of Cetrorelix Acetate INN equivalent to Cetrorelix 0.25 mg
• One ampoule containing water for injection USP
• One disposable syringe with 26G injection needle (light brown color)
• One 21G injection needle (green color).
• Two alcohol pads