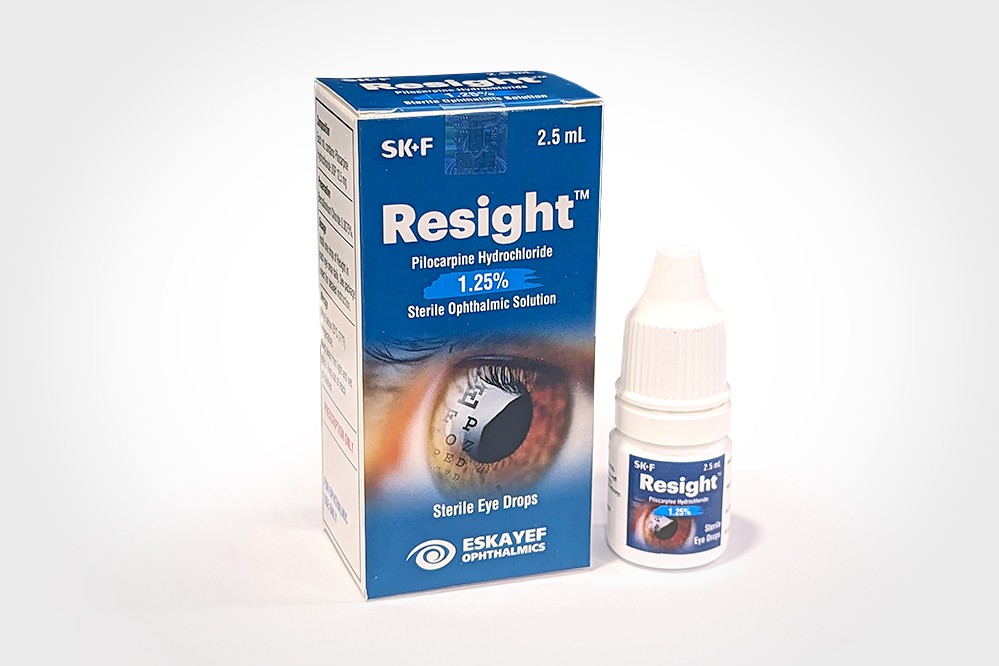
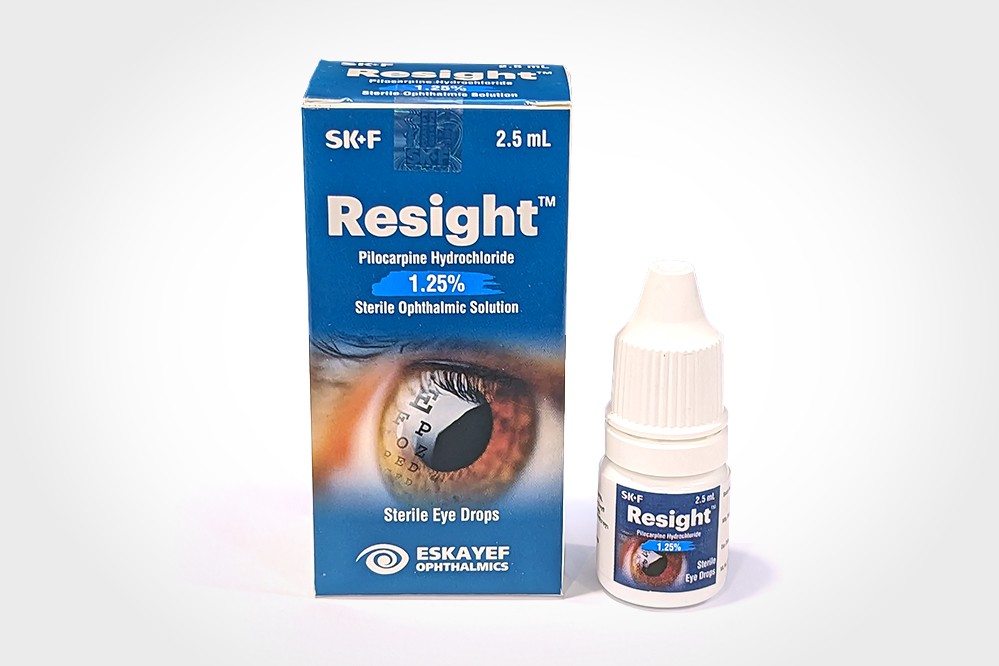


Resight
DESCRIPTION
ResightTM is a cholinergic agonist prepared as a sterile Ophthalmic Solution, containing 1.25% of Pilocarpine Hydrochloride. It is a miotic agent for topical administration to the eyes.
COMPOSITION
Each mL of ResightTM contains Pilocarpine Hydrochloride USP 12.5 mg as the active ingredient.
Preservative: Benzalkonium Chloride 0.075 mg.